How to Find Work Function Given Wavelength
If the incoming radiation has an energy equal to the work function of the metal then the frequency of the radiation is known as threshold frequency. Frequency f Wavelength λ Speed of lightc f λ c.
How Is Threshold Wavelength Related To The Work Function Quora
My guess would be to calculate the energy of the 230 nm photon and then subtract the KE of the electron from that to get the work function.
. B E hcλ - σ. The wavelength to frequency formula is given by. Wavelength Speed of the waveFrequency of the wave As mentioned above all the quantities are represented by a symbol.
Then find the wavelength of the sound wave. Firstly note down what is given in the question Frequency f 200 cycles per seconds cycless. A video solution for a problem in Modern Physics class 12th - Finding stopping potential for a given wavelength using einsteins photoelectric equationJEE M.
In mathematical terms ϕ h f K where ϕ is the work function and K is the kinetic energy of the electron. 5000 nm 5000 x 10 -9 m 5000 x 10 -7 m. With our tool you need to enter the respective value for Wavelength Work function of the surface of the metal and hit the calculate button.
The user is able to change the values of the variables waveAmplitude angleCoef and horizontalShift. Thus the work function has been calculated without having to know the threshold frequency. It is more usual to work in terms of the angular frequency ω 2πf and wave number k 2πλ so that the de Broglie relations become ω E.
Suppose I have a cathode with a work function of 3eV and an anode at a potential of 2V above the cathode. The relationship between frequency and wavelength is given as υ c λ where c is the speed of light and λ is the wavelength. Share Improve this answer answered Oct 15 2016 at 1236 ODP 4299 10 43 76 Add a comment Highly active question.
He basically gave this in the problem but with a generic work fxn W. Where λ is the wavelength and c is the speed of light in a vacuum. And I need to know whether it is possible to knock electrons off of a surface using light of a lower energy than the work function given a sufficient electrical potential.
Any electromagnetic waves frequency multiplied by its wavelength equals the speed of light. Recall that the formula for work function given incident photon wavelength is 𝑊 ℎ 𝑐 𝜆 𝐸. The work is calculated by multiplying the force by the amount of movement of an object W F d.
W a v e l e n g t h W a v e s p e e d F r e q u e n c y displaystyle Wavelength frac Wavespeed Frequency. Speed Frequency x Wavelength. Determine the photon energy if the wavelength is 650nm.
The formula for calculating wavelength is. The Photon energy formula is given by Where. M a x To find the work function of the metal we can substitute the graphs horizontal intercept value into this equation.
If the work function of the photo emitter is 4 eV find the wavelengths of radiation. The attempt at a solution. Where E is the maximum energy h is plancks constant and σ is the work function.
Nano- is 10 -9 so all you need to do is move the decimal place over 9 spots or divide by 10 9. A E hf - σ. To find the wavelength of a wave you just have to divide the waves speed by its frequency.
It also explains how to use the work function of metals to calculate the threshol. The photoelectric work function of the metal is 259 eV. But I cant quite figure out how to calculate the wavelength.
Find the number of photons striking the metal plate per square metre per second. Stopping potential V s 263 V work function Φ 4 eV 4 x 16 x 10-19 J 64 x 10-19 J speed of light c 3 x 10 8 ms Plancks constant h 663 x 10-34 Js Charge on electron e 16 x 10-19 C. The symbolic representation of the formula given above can be seen as.
Wavelength of radiation λ. Y waveAmplitude Mathsin x angleCoef horizontalShift I have to also display the wavelength on the screen. To calculate Stopping Potential you need Wavelength λ Work function of the surface of the metal phi.
You can also select the units if any for Inputs and the Output as well. A force of 10 newtons that moves an object 3 meters does 30 n-m of work. If the speed of sound is about 340 ms and the frequency of the wave crest is 200 cycles per second the low end of human hearing.
The energy of a photon is inversely proportional to the wavelength of a photon. Hcwavelength workfunction KE of Energy of electron Now workfunction is given convert it to J Then the velocity of electron is given so find out the KE of electron using 05mv2 in kgm2s2 unit Then plug in back everything in the first equation to get the wavelength of radiation in meter unit. Calculate wavelength with the wavelength equation.
λ wavelength of the light. 31 With this in mind and making use of what we already know about what the mathematical form is. Work function of silver Φ.
I got dE4425 kjmol or 734e-19 J. This chemistry video tutorial explains how the photoelectric effect works. E photon energy h Plancks constant 6626 10 34 Js c speed of the light and.
First convert nm to m. If a photon having 2eV of energy hits the cathode what happens. Mentum p a wave of frequency f and wavelength λ given by the de Broglie relations Eq.
Its typical to need to perform a unit conversion on the wavelength value in order to get it to work in the equation. A small plate of a metal work function 117 eV is placed at a distance of 2 m from a monochromatic light source of wavelength 48 x 10-7 m and power 10 watt. C f λ.
We can use this relationship to calculate the wavelength or frequency of any electromagnetic wave if. We must convert nanometers into meters so this threshold wavelength value is 3 0 0 3 0 0 1 0 n m m. Threshold wavelength λ o 4800 Å 4800 x 10-10 m speed of light c 3 x 10 8 ms Plancks constant h 663 x 10-34 Js.
Find maximum velocity of photoelectrons. The light falls normally on the plate. We need to know how the kinetic energy of the electron relates to phi or W.
In terms of wavelength to frequency the formula is given as. Solved Example on Wavelength Formula Example 1. A newton-meter is the same thing as a joule so the units for work are the same as those for energy joules.
We have Φ h ν o hcλ o-34 x 3 x 10 8 4800 x 10-10 4144 x 10-19 J-19 16 x 10-19 259 eV.

Keywords Wavelength Of Electron Keywords Glossary Of Tem Terms Jeol

The Work Function For The Caesium Atom Is 1 9 Ev Calculate A The Threshold Wavelength And B The Threshold Frequency Of The Radiation If The Caesium Element Is Irradiated With A
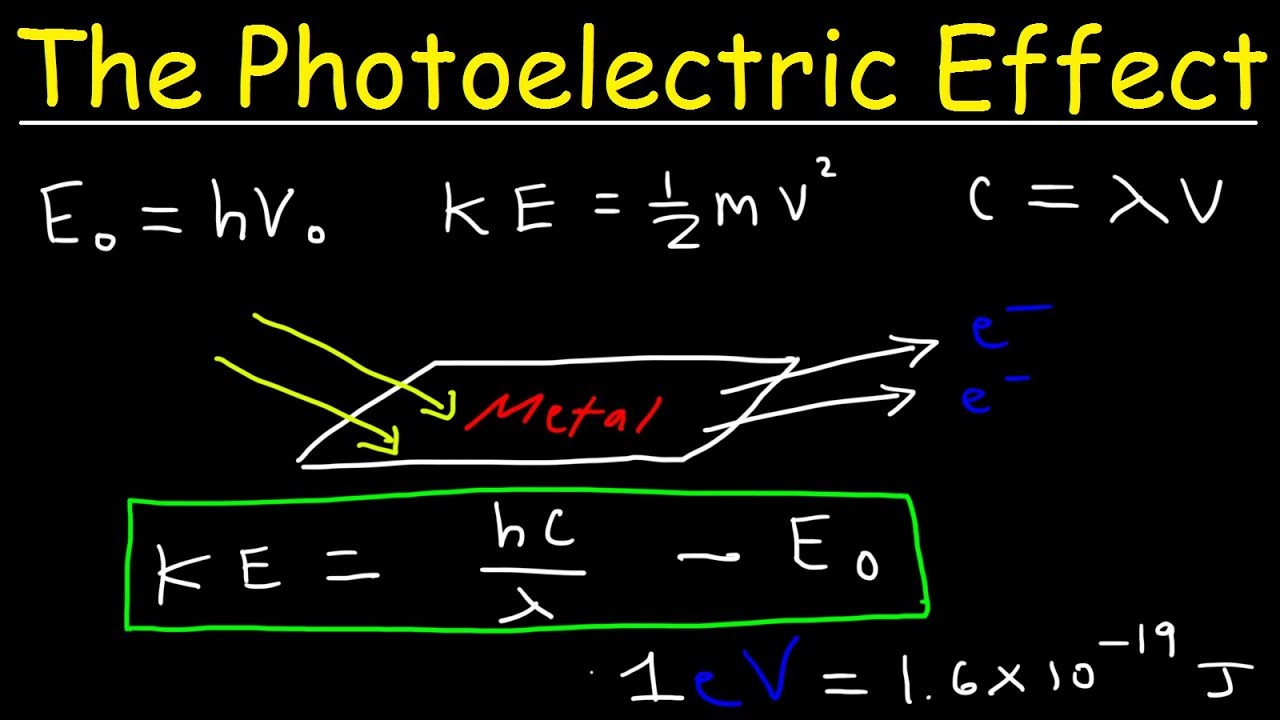
Photoelectric Effect Work Function Threshold Frequency Wavelength Speed Kinetic Energy Electr Youtube

A Metal Has A Threshold Wavelength Fo 6000 A Calculate I Threshold Frequency Ii Youtube

Work Function Formula Relation To Threshold Frequency Electrical4u

Question 14 1 Chapter Fourteen Electromagnetism Electromagnet Chapter Magnetic Field

Calculate Threshold Frequency Video Khan Academy

Eduphysics Physics Work Function Physics Kinetic Energy

Pin By Beth Froman Brown On Science Physics Physics Classroom Weather Science
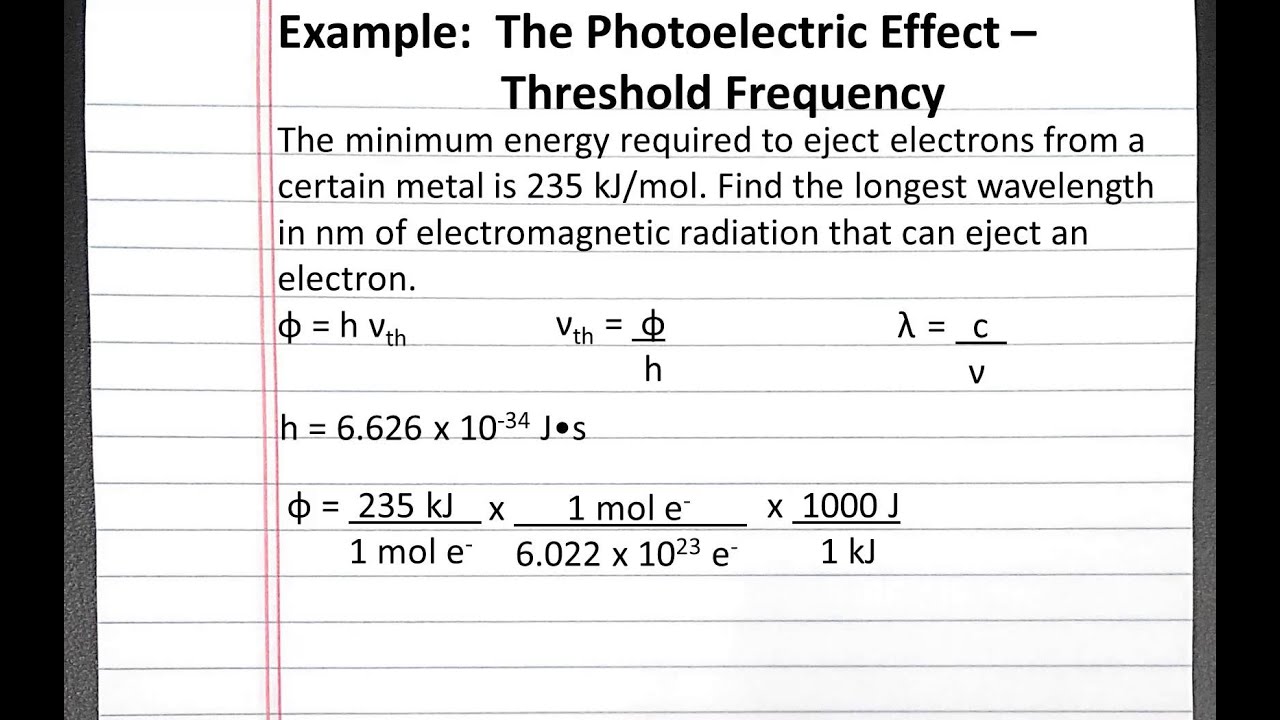
Chemistry 101 Photoelectric Effect Threshold Frequency Youtube

How Is Threshold Wavelength Related To The Work Function Quora

Question 8 1 Chapter Eight Waves Radio Wave Waves Chapter
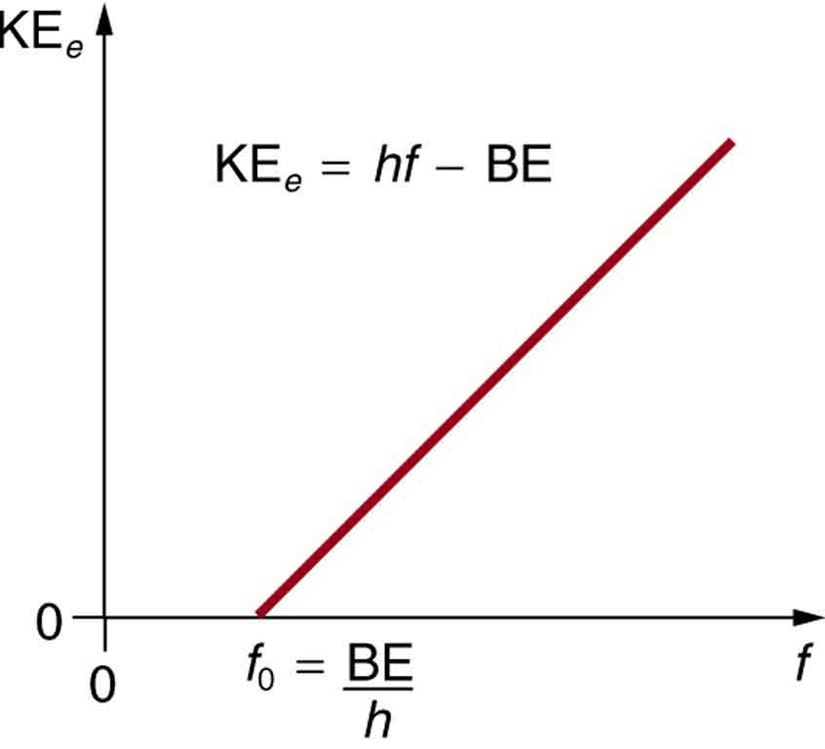
The Photoelectric Effect Physics

Planck Function Math Equations Function Math

Question 19 4 Chapter Nineteen Dawn Of Modern Physics Modern Physics Physics Work Function


Comments
Post a Comment